COVID-19 vaccine information for healthcare professionals
Updated October 13, 2021 at 10:52 amWhile there is still much to learn about COVID-19 vaccines and distribution, Children’s Health is committed to keeping referring providers informed as information becomes available.
Frequently asked questions
The following information was gathered from the CDC Coronavirus (COVID-19) website.
-
Is the COVID-19 vaccine safe?
Clinical trials investigating COVID-19 vaccines have been conducted according to rigorous standards set forth by the Food and Drug Administration (FDA) in their June 2020 guidance document, Development and Licensure of Vaccines to Prevent COVID-19. Only when the FDA determines that a vaccine meets its safety and effectiveness standards, will it make vaccines available for use in the United States by approval or emergency use authorization.
After the FDA makes its determination, the Advisory Committee on Immunization Practices (ACIP) will review available data before making vaccine recommendations to CDC. As people get vaccinated, CDC, FDA, and other federal partners will continue to conduct ongoing safety monitoring to assess for evidence of safety issues.
-
How was this vaccine developed so quickly?
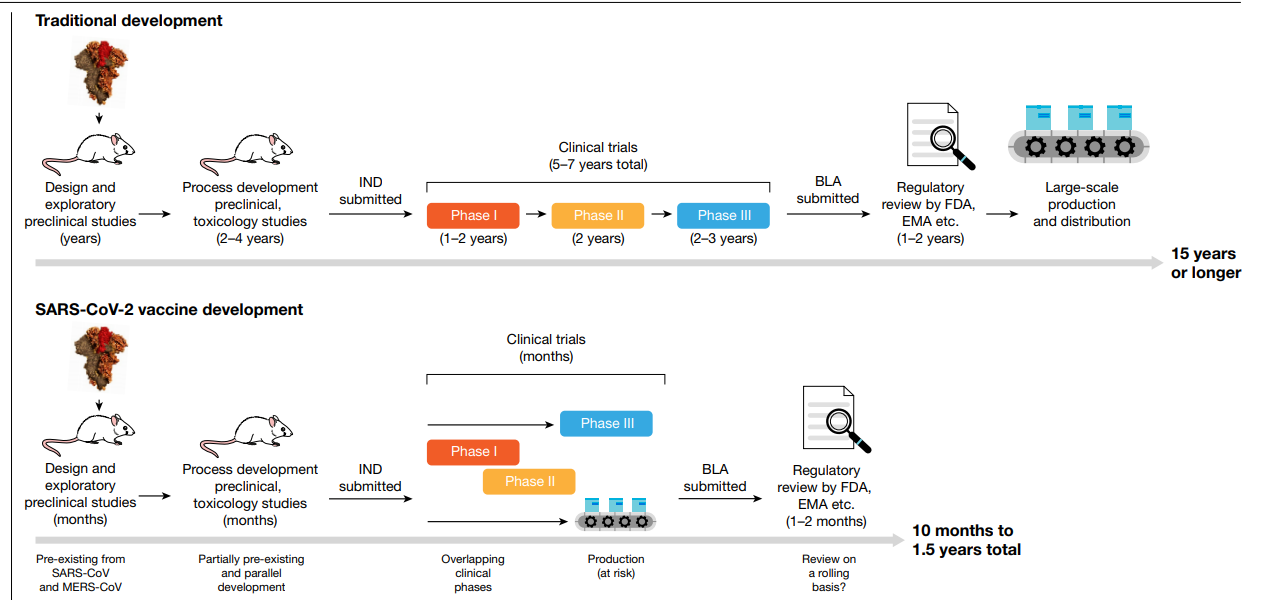
According to this research review published in Nature, SARS-CoV-2 Vaccines in Development, traditional vaccine development can take 15 years or more, starting with a lengthy discovery phase in which vaccines are designed and exploratory preclinical experiments are conducted. This is usually followed by a phase in which more formal preclinical experiments and toxicology studies are performed and in which production processes are developed. During this process an investigational new drug (IND) application is filed and the vaccine candidate then enters phase I, II and III trials. If, when phase III trials are completed, the predetermined end points have been met, a biologics license application (BLA) is filed, reviewed by regulatory agencies and finally the vaccine is licensed. After that point, large-scale production begins. Vaccine development for SARS-CoV-2 is following an accelerated timeline. Because of knowledge gained from the initial development of vaccines for SARS-CoV and MERS-CoV, the discovery phase was omitted. Existing processes were adopted, and phase I/II trials were started. Phase III trials were initiated after the interim analysis of phase I/II results, with several clinical trial stages running in parallel. In the meantime, vaccine producers have started the large-scale production of several vaccine candidates, at risk. The exact pathway by which these vaccine candidates will be licensed—for example, through an initial emergency use authorization—is not yet clear.
-
When will the COVID-19 vaccine be available?
During emergencies, the FDA has the ability to grant Emergency Authorization Use (EAU), which allows the FDA to make a vaccine available if there is evidence that strongly suggests that patients have benefited from the vaccine in clinical trials. The FDA granted EAU for the Moderna and Pfizer vaccines in mid-December.
-
Who will receive the vaccine first?
It is likely there will not be enough vaccinations available for all adults right away, but supplies will increase over time. The Advisory Committee on Immunization Practices (ACIP), a federal advisory committee made up of medical and public health experts who develop recommendations on the use of vaccines in the U.S. public, took into consideration a number of factors when making recommendations on who should receive the vaccine first.
On Thursday, Dec. 3, the CDC approved the advisory panel's recommendations that those first in line to receive COVID-19 vaccines should be health care workers and residents of long-term care facilities.
-
Is the COVID-19 vaccine safe for children?
In early clinical trials for various COVID-19 vaccines, only non-pregnant adults participated. The trial run by Pfizer and BioNTech was initially open to people 18 or older. Pfizer started including participants as young as 16 in September and last month, launched a new trial on children as young as 12. Currently, the CDC does not recommend COVID-19 vaccines for children. The groups recommended to receive the vaccines could change in the future as clinical trials progress. Pfizer's vaccine was granted emergency use for people ages 16 and older and Moderna's is only available for people ages 18 and older. Safety is our top priority, and Children’s Health will not offer a vaccine to patients that we do not believe to be scientifically safe and effective.
-
Can I get COVID-19 from the vaccine?
You cannot contract COVID-19 from the vaccine. The Pfizer and Moderna vaccines use only a gene from the virus while other vaccines being studied use inactivated virus, none of which cause COVID-19.
Vaccines currently in clinical trials in the United States won’t cause you to test positive on viral tests, which are used to see if you have a current infection. However, if your body develops an immune response, which is the goal of vaccination, there is a possibility you may test positive on some antibody tests. Experts are currently looking at how the COVID-19 vaccination may affect antibody testing results.
-
If I have had COVID-19, should I get the vaccine?
There is not enough information currently available to say if or for how long after infection someone is protected from getting COVID-19 again; this is called natural immunity. Early evidence suggests natural immunity from COVID-19 may not last very long, but more studies are needed to better understand this. Until we have a vaccine available and the Advisory Committee on Immunization Practices makes recommendations to CDC on how to best use COVID-19 vaccines, the CDC cannot comment on whether people who had COVID-19 should get a COVID-19 vaccine.
-
Should we continue to wear face masks?
Yes. Once approved, the COVID-19 vaccine will take months to distribute and administer. Stopping a pandemic requires using all the tools available. Vaccines work with your immune system so your body will be ready to fight the virus if you are exposed. Other steps, like covering your mouth and nose with a mask and staying at least 6 feet away from others, help reduce your chance of being exposed to the virus or spreading it to others. Everyone should continue wearing face masks, practicing hand hygiene and social distancing until enough vaccine is manufactured and distributed, until we know how long a vaccine will protect us, and until our community shows levels of minimal spread.
-
How many doses of the COVID-19 vaccine are required?
Both the Pfizer and Moderna vaccine require two doses on a carefully calibrated schedule (for Pfizer a second dose 21 days later; for Moderna 24-28 days later). Both doses are required to ensure full effectiveness of the vaccine. The vaccines are not interchangeable – both doses must be from the same manufacturer.
-
What are the side effects of the COVID-19 vaccine?
Responses to the vaccine will vary by person. The Pfizer vaccine data demonstrates that it was well tolerated across all populations with over 43,000 participants enrolled. Only 3.8 percent of participants reported fatigue and 2 percent reported headaches. Scientists anticipate that other COVID-19 vaccines may cause mild flu-like responses — including sore arms, muscle aches and fever.
- Provider Resources for COVID-19 Vaccine Conversations with Patients
- 10 Things Healthcare Professionals Need to Know about U.S. COVID-19 Vaccination Plans
- Governor Greg Abbott 's COVID-19 vaccine allocation process
- The New York Times Coronavirus Drug and Treatment Tracker
- CDC General COVID-19 Vaccine Information
- FDA COVID-19 Vaccine Information
- Texas COVID-19 Vaccination Plan
- DSHS Immunizations Unit: COVID-19 Vaccine Information
- Pfizer Coronavirus Vaccine
- Moderna COVID-19 Vaccine
-
[VIDEO] How we got here - The historic development of COVID-19 vaccines
The fast development of the COVID-19 vaccines has been unprecedented. In this video, Jeffrey Kahn, M.D., Division Director of Infectious Diseases, Medical Director of Research at Children’s Health℠ and Professor at UT Southwestern:
- Outlines how the COVID‑19 vaccines were developed so quickly
- Gives an overview of the mRNA technology used
- Discusses the safety and efficacy of the current vaccines granted Emergency Use Authorization by the FDA
MuteCurrent Time 0:00/Duration Time 0:00Loaded: 0%0:00Progress: 0%0:00Progress: 0%Stream TypeLIVERemaining Time -0:00Playback Rate1Chapters- Chapters
Descriptions- descriptions off, selected
Subtitles- subtitles off, selected
Captions- captions settings, opens captions settings dialog
- captions off, selected
Audio TrackThis is a modal window.
Caption Settings DialogBeginning of dialog window. Escape will cancel and close the window.This is a modal window. This modal can be closed by pressing the Escape key or activating the close button.
This is a modal window. This modal can be closed by pressing the Escape key or activating the close button.
This video was presented at a Children’s Health town hall for providers in September of 2021.